Transforming Catheter Manufacturing: The ENKI Extrusion Innovation Initiative
Article
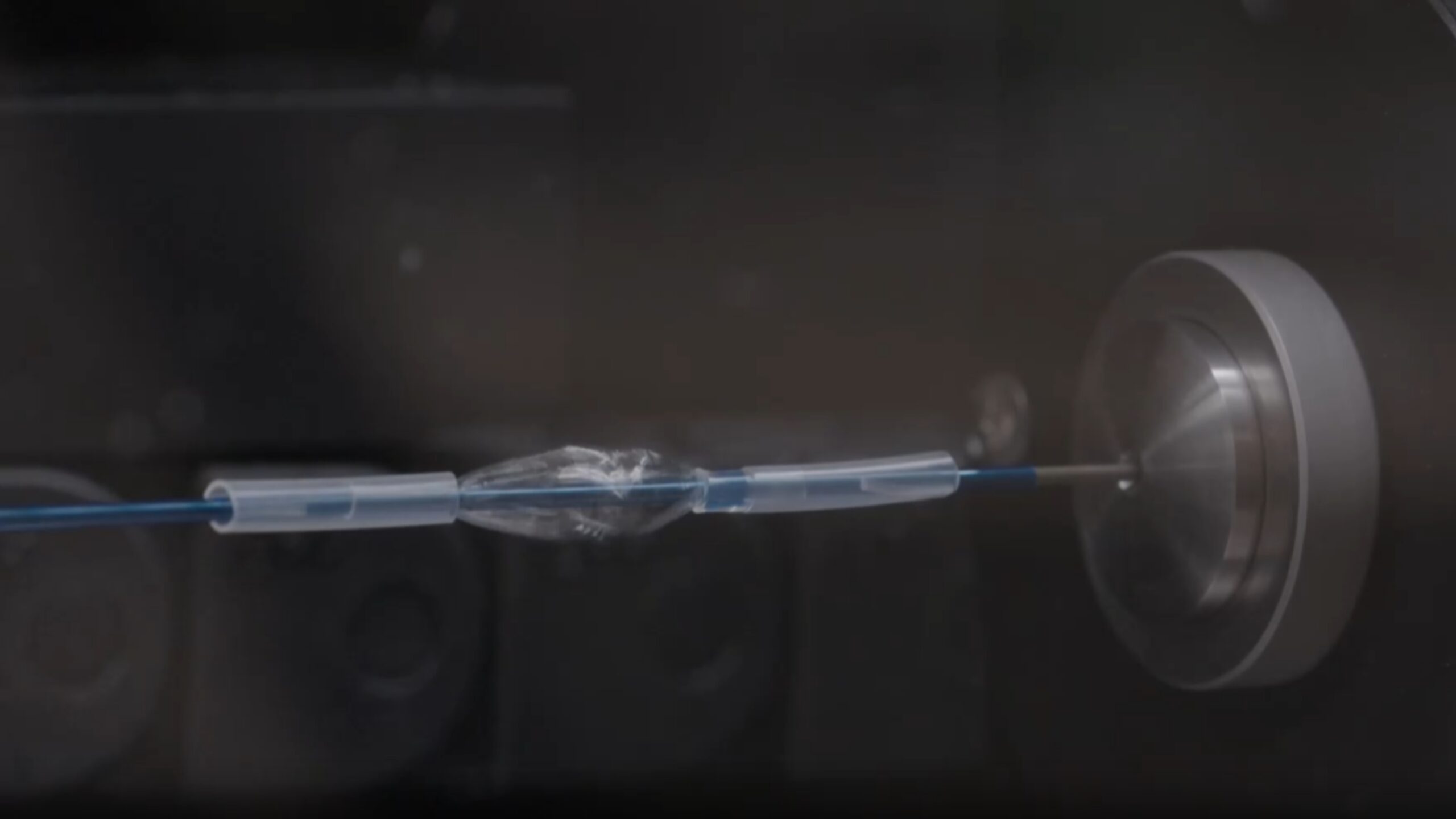
The Extrusion use case
In the realm of catheter production, ENKI stands at the forefront of innovation, particularly through the DAT4.ZERO project. The Extrusion use case is poised to redefine operational efficiencies and product quality, marking a significant leap forward in the industry.
The core of the ENKI business case lies in addressing critical challenges such as reducing setup duration and minimizing scraps during the setup phase. Through predictive analytics, the project aims to forecast parameters for future product developments, thereby optimizing production
planning. This strategic initiative is not just about improving production metrics; but marks it’s the beginning of a new era of manufacturing excellence.
The benefits of this endeavor are manifold. By integrating advanced sensors and employing artificial intelligence, ENKI is set to experience a reduction in scraps and an increase in production rates. This translates to offering higher quality products at lower costs and with shorter lead times for the development of new products. Moreover, the introduction of an augmented reality visor promises not only enhanced product quality but also improved working conditions for operators.
However, the journey is not devoid of challenges. The installation of new sensors in place of existing ones carries the risk of potential production line stops and additional maintenance if they prove ineffective. Such hurdles underscore the importance of a carefully planned and executed
implementation strategy.
The next steps for the ENKI business case are as strategic as they are transformative. Post-project, the focus will shift towards providing substantial support to the production supervisor, reducing workload, and enhancing shop floor operations with AR visors to alleviate eye strain, thus offering
health benefits. The application of feedforward process parameters, such as speed, pressure, and temperature, will facilitate the extrusion process, enabling even novice operators to make informed decisions.
In conclusion, the ENKI business case within the DAT4.ZERO project represents a groundbreaking initiative that not only addresses existing production inefficiencies but also sets a new standard for quality and operational excellence in the medical device manufacturing sector. With its forward-thinking approach and commitment to leveraging cutting-edge technologies, ENKI is poised to achieve remarkable improvements in productivity, quality, and worker well-being, charting a course for future expansions and sustained competitiveness.
